Techniques
1. In vitro motility assay
 In the in vitro motility assay single cytoskeletal filaments (actin filaments or microtubules) are visualized as they are propelled forth on a surface coated with motor proteins (myosin or kinesin, respectively). To visualize the filaments, the diameter of which falls far below the diffraction limit, they are fluorescently labeled. Filaments are visalized in an epi- fluorescence microscope using sensitive cameras.
In the in vitro motility assay single cytoskeletal filaments (actin filaments or microtubules) are visualized as they are propelled forth on a surface coated with motor proteins (myosin or kinesin, respectively). To visualize the filaments, the diameter of which falls far below the diffraction limit, they are fluorescently labeled. Filaments are visalized in an epi- fluorescence microscope using sensitive cameras.
In our setup a microchannel-plate intensified CCD camera (VideoScope) is mounted on a Zeiss Universal epifluorescence microscope. The video is recorded in S-VHS, Hi8mm or Digital8 format, and filament movement is analyzed off-line with NIH-Image software using custom-written macro algorithms. For the motility assay macros click here...
2. Force-measuring optical tweezers
 In optical tweezers momentum is exchanged between the photons in a powerful laser beam and a refractile microscopic bead that functions as a molecular handle. As a result, the bead - and hence the molecule captured by the bead - can be mechanically manipulated. For more info...
In optical tweezers momentum is exchanged between the photons in a powerful laser beam and a refractile microscopic bead that functions as a molecular handle. As a result, the bead - and hence the molecule captured by the bead - can be mechanically manipulated. For more info...
Our instrument is a dual-beam, counter-propagating optical tweezers setup that measures forces by monitoring light momentum change. In our setup simultaneous fluorescence imaging is available.
3. Single-molecule force spectroscopy
 Single-molecule force spectroscopy is a novel method in which surface-adsorbed molecules are stretched away from the surface with an AFM cantilever. The force generated in the molecule is measured by monitoring the bending of the cantilever. From the force versus extension functions of the molecule its elasticity can be characterized. In addition, by following transitions in the force curve intra- (e.g., domain unfolding) or intermolecular (e.g., ligand rupture) events can be explored. By plotting the characteristic force as a function of loading rate various kinetic parameters of the process may be obtained in what became called"dynamic force spectroscopy."
Single-molecule force spectroscopy is a novel method in which surface-adsorbed molecules are stretched away from the surface with an AFM cantilever. The force generated in the molecule is measured by monitoring the bending of the cantilever. From the force versus extension functions of the molecule its elasticity can be characterized. In addition, by following transitions in the force curve intra- (e.g., domain unfolding) or intermolecular (e.g., ligand rupture) events can be explored. By plotting the characteristic force as a function of loading rate various kinetic parameters of the process may be obtained in what became called"dynamic force spectroscopy."
Our instrument is an Asylum Research Molecular Force Probe-1D (MFP-1D) mounted on a custom-built inverted microscope housed in an acoustically and vibrationally isolated workstation. With an add-on hot-plate, temperature-dependent force spectroscopy measurements can be carried out.
4. Atomic force microscopy
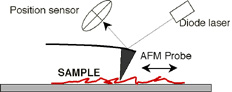 In atomic force microscopy (AFM) a narrow tip - which is located at the end of a flexible cantilever - scans the sample surface. Attractive and repulsive forces between the atoms of the cantilever tip and the sample result in vertical deflections of the cantilever. The deflections are measured from the changes in the position of a laser beam projected on the back of the cantilever. Our instrument is a state-of-the-art MFP-3D AFM from Asylum Research. The instrument is housed in an acoustically and vibbrationally isolated workstation. The instrument is mounted on a fully motorized inverted microscope (Olympus IX81), and is combined with TIRF microscopy (see below) for the simultaneous topography scanning and fluorescence imaging of single molecules.
In atomic force microscopy (AFM) a narrow tip - which is located at the end of a flexible cantilever - scans the sample surface. Attractive and repulsive forces between the atoms of the cantilever tip and the sample result in vertical deflections of the cantilever. The deflections are measured from the changes in the position of a laser beam projected on the back of the cantilever. Our instrument is a state-of-the-art MFP-3D AFM from Asylum Research. The instrument is housed in an acoustically and vibbrationally isolated workstation. The instrument is mounted on a fully motorized inverted microscope (Olympus IX81), and is combined with TIRF microscopy (see below) for the simultaneous topography scanning and fluorescence imaging of single molecules.
5. Total internal reflection fluorescence microscopy
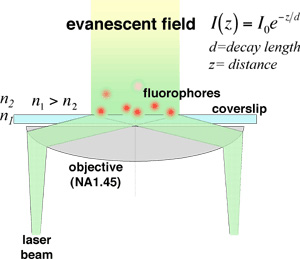 In total internal reflection fluorescence (TIRF) microscopy a laser beam arrives from a high refractive index medium (glass) to a boundary with a low refractive index medium (aqueous solution) at an angle that exceeds the so caled critical angle. At this boundary total reflection occurs, but a so called "evanescent field" is formed in the lower refractive index medium. The intensity of the field decays expponentially with the distance and its height is comparable with the wavelength of the light used. As a result, a layer of fluorofores can be excited in such a way that the background intensity remains remarkably low. It is also worth noting that since the field decays in t ashort distance, there is no interaction with the AFM cantilever, in contrast to other imaging techniques such as confocal or far-field fluorescence microcopy.
In total internal reflection fluorescence (TIRF) microscopy a laser beam arrives from a high refractive index medium (glass) to a boundary with a low refractive index medium (aqueous solution) at an angle that exceeds the so caled critical angle. At this boundary total reflection occurs, but a so called "evanescent field" is formed in the lower refractive index medium. The intensity of the field decays expponentially with the distance and its height is comparable with the wavelength of the light used. As a result, a layer of fluorofores can be excited in such a way that the background intensity remains remarkably low. It is also worth noting that since the field decays in t ashort distance, there is no interaction with the AFM cantilever, in contrast to other imaging techniques such as confocal or far-field fluorescence microcopy.
In our setup we utilize objective-based TIRF. Two lasers are used: a 50 mW, 532 nm YAG laser and a 15 mW, 488 nm diode laser. The two beams are combined using a long-pass dichroic mirror and projected via a beam-stering mirror system into a motorized iverted microscope (Olympus IX81). The microscope is equipped with a TIRF adaptor, by the help of which the objective-based TIRF can be adjusted. Images are collected via the bottom port of the microscope by either a MCP-intensified CCD cameraor an avalanche photodiode (APD).
6. Molecular biology
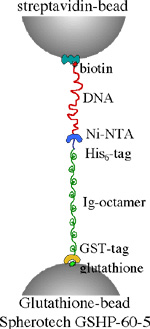 We utiize recombinant techniques to modify and generate the molecular specimens of our interest. Heterologous expression systems (primarily E. coli) are used to express the proteins of interest. Cysteine residues are inserted by using site-directed mutagenesis, for the purpose of subsequent fluorescent modification or for the insertion of molecular handles. Proteins,which carry His-tag, are purified using Ni-chelate columns. An example of a molecular system with various handles is shown below.
We utiize recombinant techniques to modify and generate the molecular specimens of our interest. Heterologous expression systems (primarily E. coli) are used to express the proteins of interest. Cysteine residues are inserted by using site-directed mutagenesis, for the purpose of subsequent fluorescent modification or for the insertion of molecular handles. Proteins,which carry His-tag, are purified using Ni-chelate columns. An example of a molecular system with various handles is shown below.



